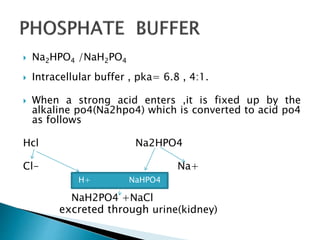
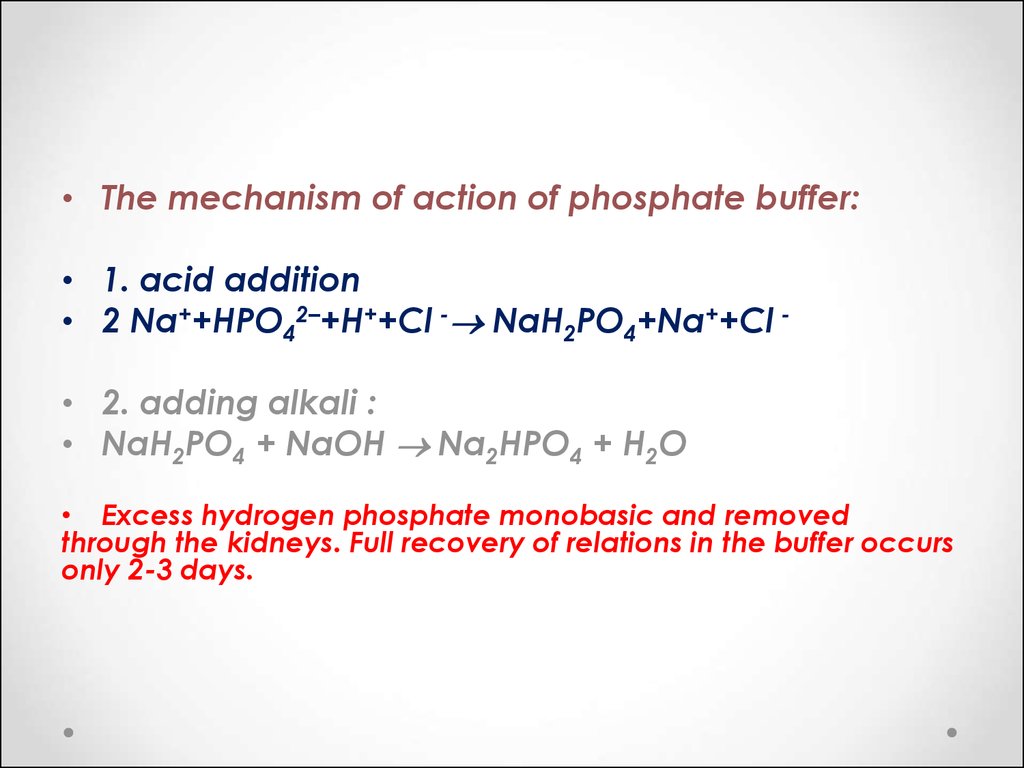
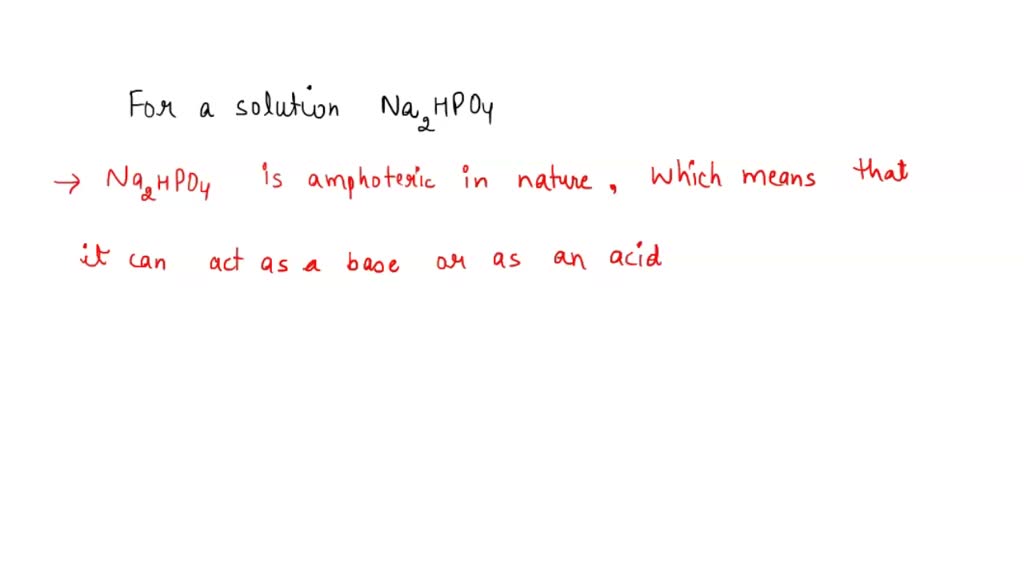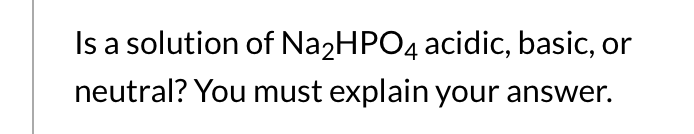Calculate the pH of a buffer solution obtained by dissolving 25.0 g of KH2PO4(s) and 38.0 g of Na2HPO4(s) in water and then diluting to 1.00 L. | Homework.Study.com
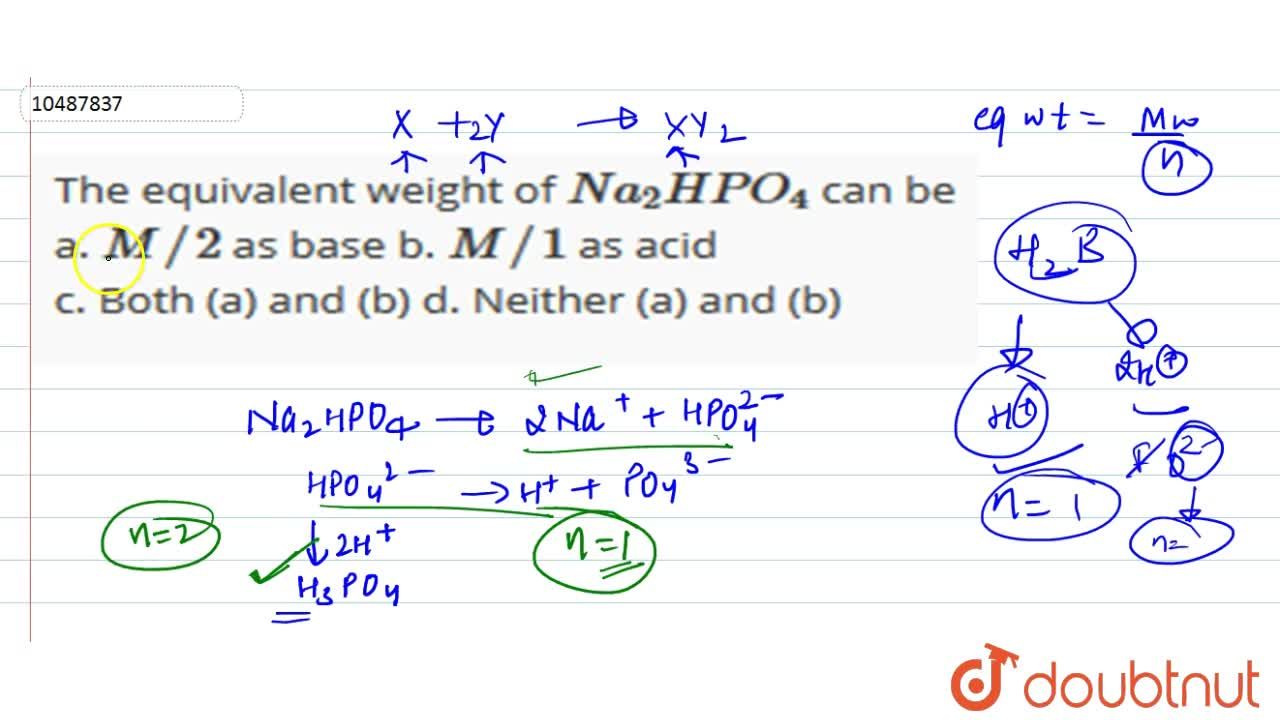
The equivalent weight of Na(2) HPO(4) can be a. M//2 as base b. M//1 as acid c. Both (a) and (b) d. Neither (a) and (b)
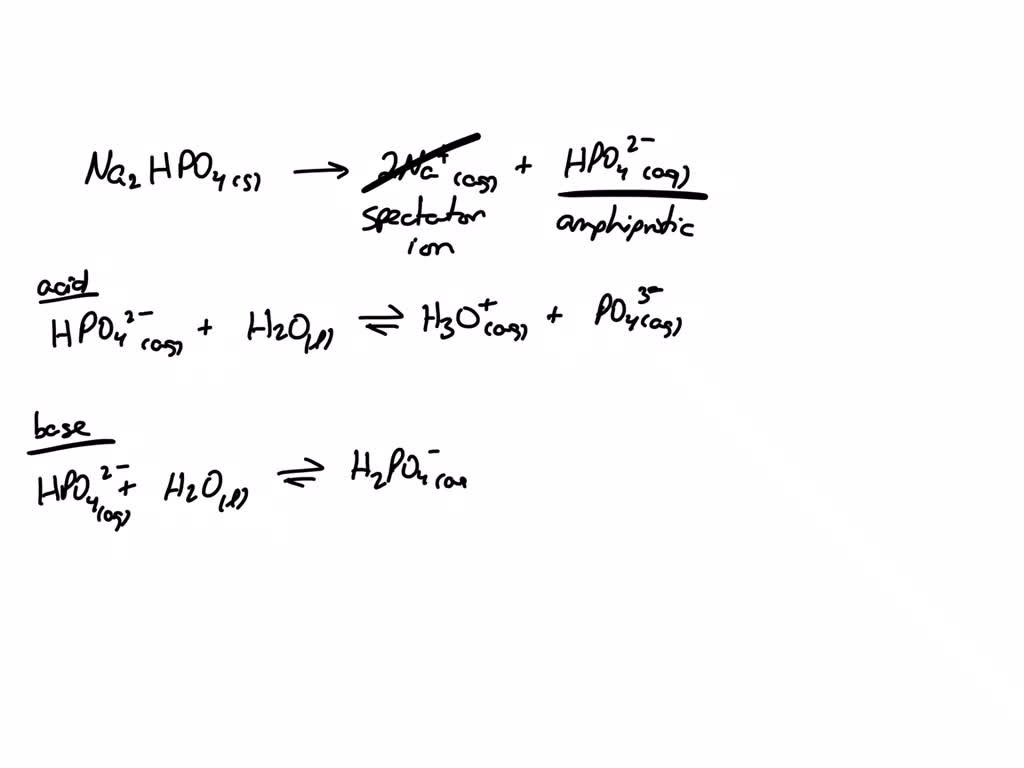
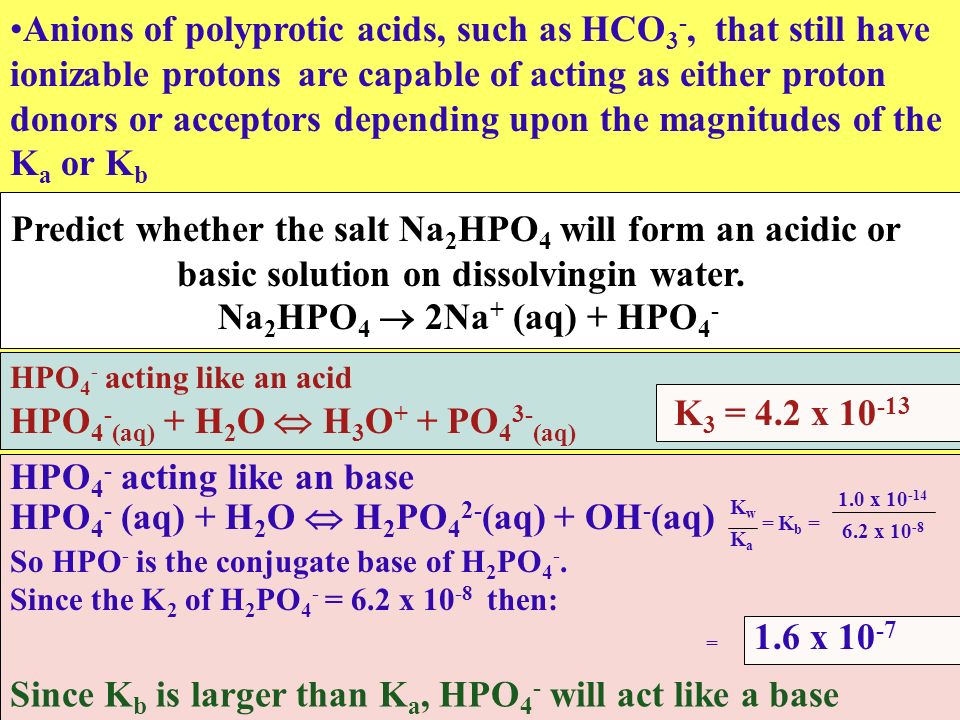
SOLVED: Predict whether the salt Na2HPO4 forms an acidic solution or a basic solution when dissolved in water.

OneClass: 2. In the following reactions, label the acid, base, conjugate acid and conjugate base. (4 ...

If 2.5 moles each of H3PO4,NaH2PO4,Na2HPO4 and Na3PO4 are mixed together to form an aqueous solution, then the resulting pH is:Given values of Ka are: Ka1 = 10^-3 Ka2 = 10^-7 Ka3 = 10^-13
Q. The equivalent mass of H3PO4 (Molecular weight = 98 g/mol) and Na2HPO4 (Molecular weight = 142 g/mol) in the reaction are respectively : H3PO4 + 2NaOH → Na2HPO4 + 2H2O (1) 49, 142 (2) 49, 71 (3) 98, 71 (4) 98, 142