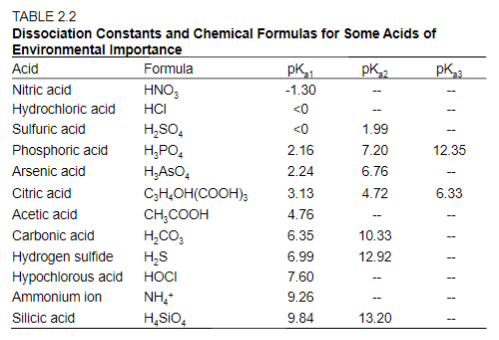
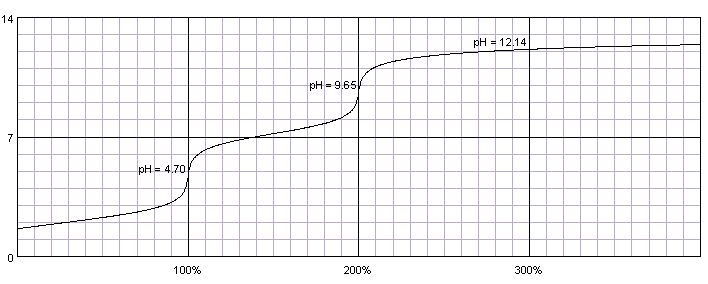
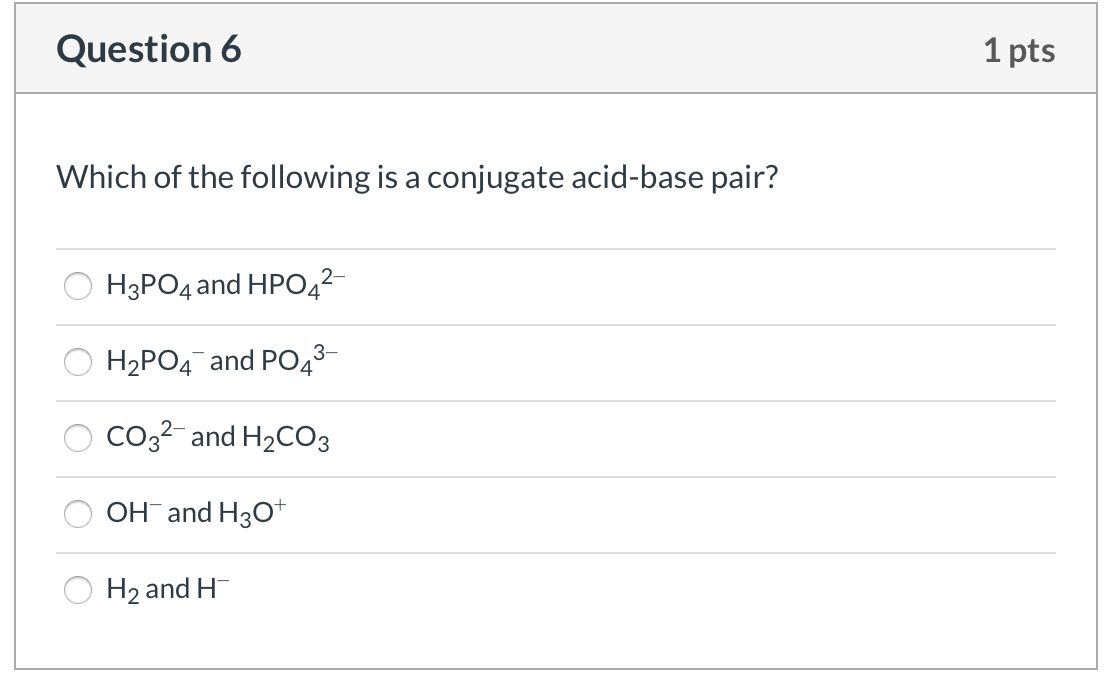
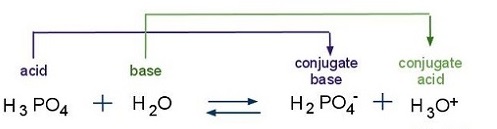
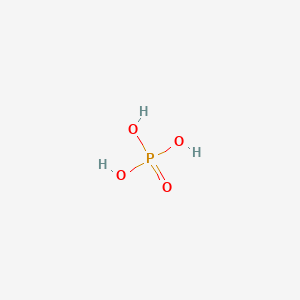
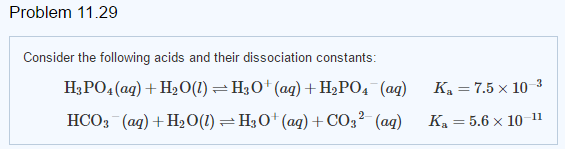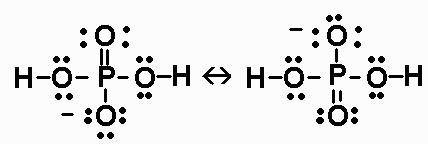SOLVED: Compare the conjugate bases of these three acids Acid 1: carbonic acid H-COz Acid 2: phosphoric acid . HzPO4 Acid 3: hydrogen sulfite . HSO3 WVhat is the formula for the
![Orthophosphoric Acid (H3PO4) [Phosphoric Acid] - Structure, Formula, Synthesis, Properties, Uses and FAQs of Orthophosphoric acid. Orthophosphoric Acid (H3PO4) [Phosphoric Acid] - Structure, Formula, Synthesis, Properties, Uses and FAQs of Orthophosphoric acid.](https://cdn1.byjus.com/wp-content/uploads/2018/11/phosphoric-acid-structure.png)
Orthophosphoric Acid (H3PO4) [Phosphoric Acid] - Structure, Formula, Synthesis, Properties, Uses and FAQs of Orthophosphoric acid.

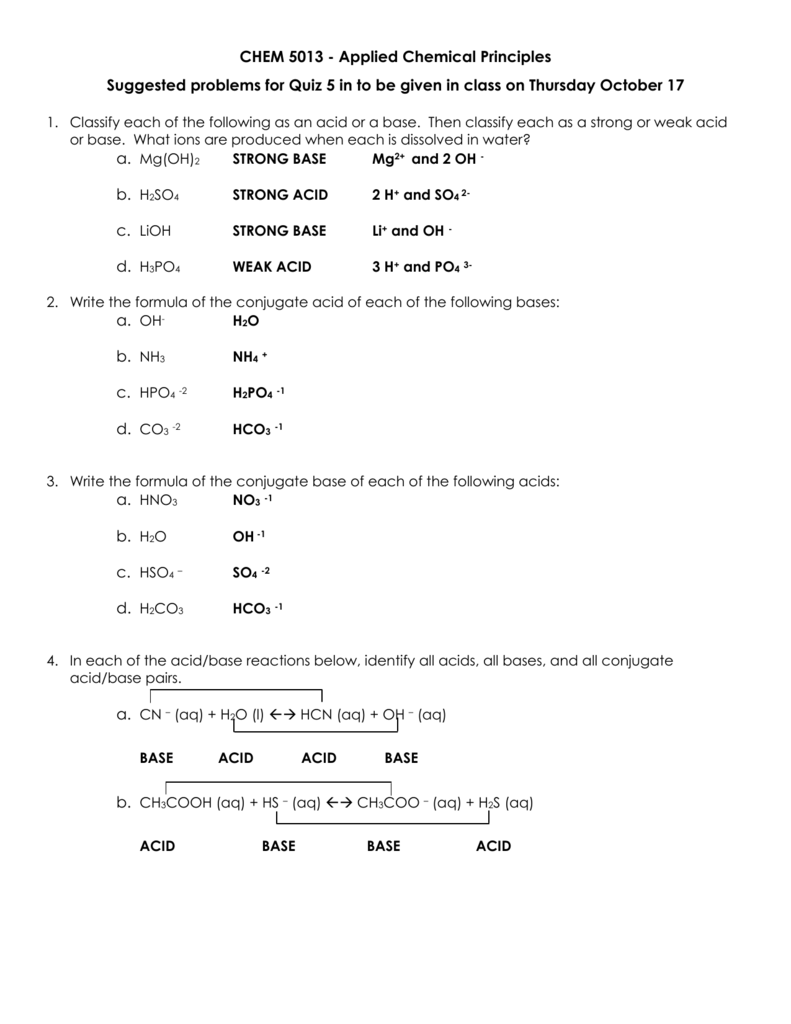
Classify the following as acid (or) base using Arrhenius concept HNO3 (ii) Ba(OH)2 (iii) H3PO4 (iv) CH3COOH

15) Circle one and tell whya. CH3COOH is a n) (acid, base, salt)b. NH4Cl is a(n) (acid, base, salt)c. - Brainly.com
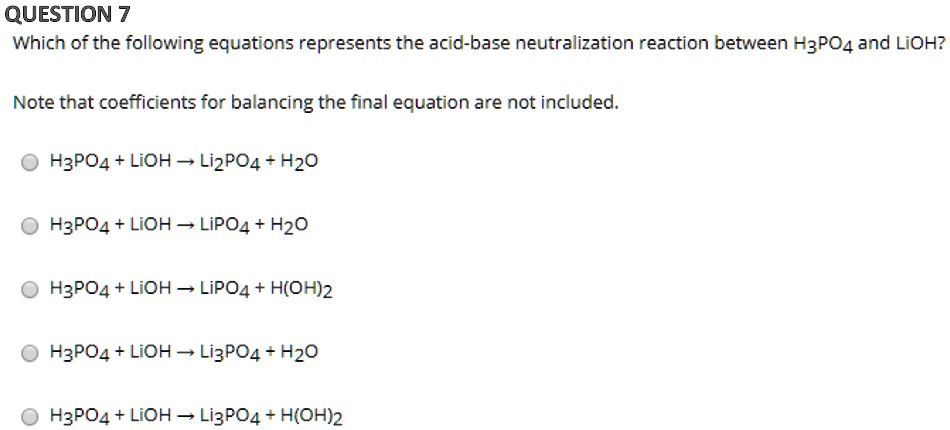
SOLVED: QUESTION 7 Which of the following equations represents the acid-base neutralization reaction between H3PO4 ad LiOH? Note that coefficients for balancing the final equation are not included: H3PO4 + LiOH LizPO4 -

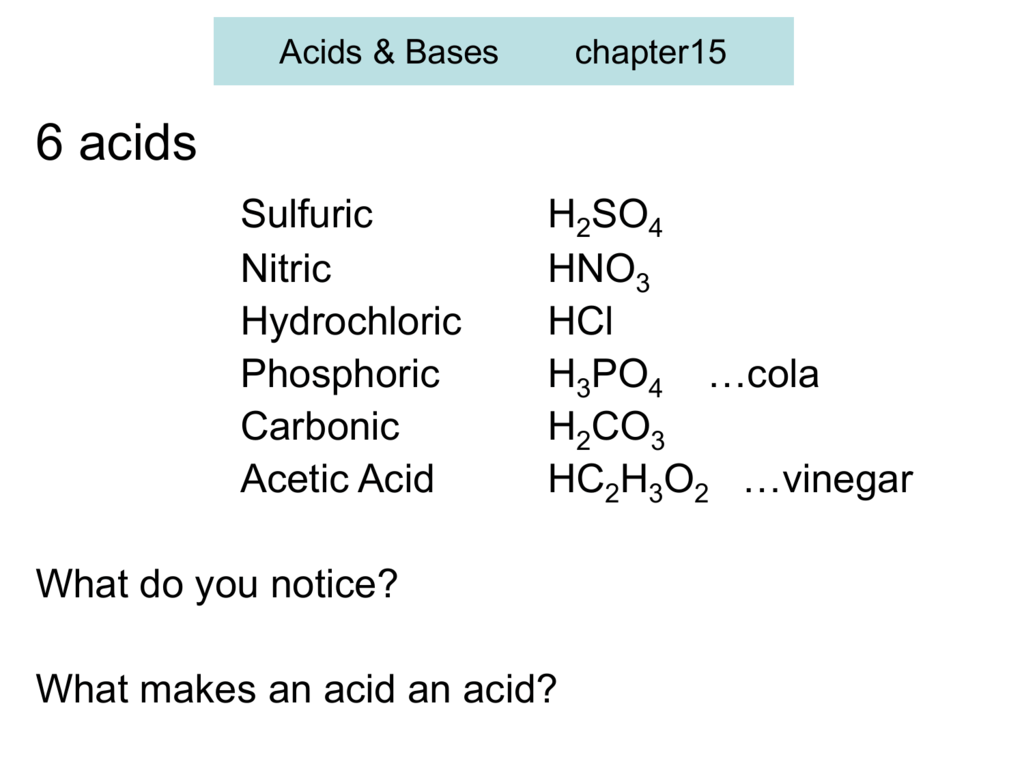
Acid-Base Test Review Definitions of Acids and Bases 1. Which of the following are Arrhenius acids? a. H2O b. H3PO4 c. NH3 d. H2SO3 2. Which of the. - ppt download